Abstract
Introduction
Acute myeloid leukemias (AMLs) are characterized by suppressed cell death pathways which promote leukemic blast survival. TP53 acts as a tumor suppressor gene in AML and is found mutated or deleted in 10-15% of patients. In a majority of cases though, TP53 is wild-type. Other mechanisms including MDM2 over-expression lead to reduced TP53 activity. MDM2 acts as a negative regulator by direct binding of TP53 and mediating TP53 degradation through ubiquitination. MDM2 itself is a transcriptional target of TP53 as a negative feedback mechanism limiting the function of TP53. Small molecule inhibition of MDM2 , blocking its ability to bind TP53, can activate TP53 and trigger cell cycle arrest and apoptosis through increased transcription of TP53 target genes. Increased MDM2 expression has been observed in hematologic malignancies including AML, providing rationale for clinical trials with MDM2 inhibitors. These agents such as RG7388 and AMG232 have shown efficacy as monotherapy and in combination. However, these agents have also exhibited toxicity and have yet to demonstrate sufficient benefit for their approval. To create more effective agents against MDM2, we have developed an MDM2 degrader XY-27 that functions as a proteolysis-targeting chimera (PROTAC). Based on relatively higher expression in AML compared to other cancer types, we selected VHL as the E3 ubiquitin ligase target for XY-27 , as this may improve specificity and potency in AML.
Results
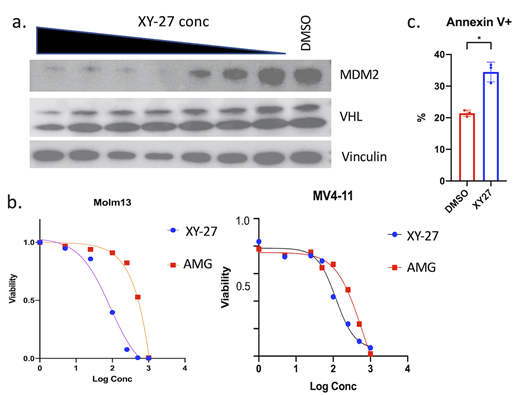
The PROTAC degrader XY-27 concurrently binds MDM2 and VHL, and by bringing these targets in proximity, VHL can then ubiquitinate MDM2, leading to its degradation by the proteasome. XY-27 can mediate degradation of MDM2 in a concentration dependent manner in the U937 leukemia cell line (Fig 1a). MDM2 degradation with XY-27 is blocked by proteasome inhibition and competitive binding of the VHL ligand. A control compound, which only differs in that it cannot bind to VHL, lacks degrader activity. Although MDM2 is itself an E3 ligase, VHL expression is not appreciably changed with XY-27 (Fig 1a).
Treatment with XY-27 leads to apoptosis and decreased proliferation of leukemia cell lines in a TP53 dependent manner. Inhibition of MDM2 leads to up-regulation of TP53 and in TP53 wild-type cells, downstream targets CDKN1A (p21) and PUMA. MDM2 is also up-regulated through a feedback mechanism. XY-27 demonstrated greater potency than the MDM2-binding inhibitor AMG232 in the MOLM13 and MV4-11 leukemia cell lines (Fig 1b). Treatment with XY-27 led to higher levels of TP53 and p21 protein than with AMG232. CRISPR-mediated knock-out of VHL leads to reduced XY-27 potency. XY-27 also shows efficacy when combined with other chemotherapeutic agents such as azacytidine and cytarabine. In a long-term co-culture model with an OP9 feeder layer, XY-27 was capable of inducing apoptosis in primary patient AML samples (Fig 1c).
Conclusion
We describe a new MDM2 PROTAC, XY-27 that demonstrates TP53 dependent activity against leukemia cells. It also demonstrates increase potency compared to an MDM2 binding inhibitor. Utilization of the PROTAC system has potential advantages through selection of the VHL E3 ubiquitin ligase. Because of negative feedback mechanisms involving TP53 and MDM2, direct binding inhibitors of MDM2 may be limited in activity through continued accumulation of MDM2. PROTAC degraders have catalytic activity and may overcome this inhibition by continued degradation of the target MDM2, and thus achieve greater TP53 activity.
Figure 1. Activity of the MDM2-PROTAC XY-27 in leukemia. (a) Western blot from treatment of U937 leukemia cells with XY-27 for 24 hrs, at various concentrations (5 nM to 1 μM), resulting in the degradation of MDM2. (b) Dose response curves from treatment of MOLM13 and MV4-11 cell lines with XY-27 (blue) and AMG232 (red) for 48 hrs, demonstrating greater potency of XY-27. (c) Induction of apoptosis in primary AML cells treated with XY-27 at 1μM using a co-culture system for 3 days. *p<.05
Hoffman: Protagonist Therapeutics, Inc.: Consultancy; AbbVie Inc.: Other: Data Safety Monitoring Board, Research Funding; Novartis: Other: Data Safety Monitoring Board, Research Funding; Kartos Therapeutics, Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal